Do you have what it takes to grow your own crystal? Of QUARTZ you do!
Why not assemble your own science club and try growing your own salt crystal? All you have to do is grow the biggest and brightest salt crystals following the recipe below, adding in your own experimental materials to make them spectacular! It’s as easy as 1,2,3!
The Recipe
Here’s a basic recipe (don’t forget to add your own ingredients such as food colouring to make the crystals big and bright):
1. Boil some water in a pan and slowly add salt. Please be careful and ask an adult to help you with this stage (unless you are an adult yourself).
At first, the salt will dissolve very quickly, but as you continue to add more it will eventually collect at the bottom of the pan which means the solution has become super-saturated.
2. Once you have a super-saturated solution, pour this into a glass container so you can easily see what is going on inside. Place it somewhere safe at room temperature.
3. Go away and do something that takes more than a day. For example, you could build a magnificent city like Rome, count to 86401, or walk from Lincoln to Coventry.
4. Go back to look at your solution. You should already see crystals being formed, but they will probably not be full-size yet.
5. Repeat steps 3 and 4 until you have some nice big crystals!
6. Send us a photo! Make sure to use the #UoLCrystals.
Additional Tips
- It might be a good idea to give the nucleation a bit of a head-start by suspending something with a rough surface (like some string) in your solution during steps 3-5. Nucleation is more likely to occur in nooks and crannies of a surface than in the solution itself.
- You may want to try avoiding the nucleation stage altogether and introduce ‘seed crystals’ to your cooled, super-saturated solution. Perform steps 1-5 above and choose a crystal with a large flat surface as your ‘seed’. Make a new super-saturated salt solution and add your seed immediately before step 3 once the solution has cooled to room temperature.
- Impurities in the original salt can significantly alter the process. This can make the resulting crystals look more vibrant but can also hinder their formation. Experiment!
- The shape and surface of the container may make a difference, so you may want to experiment with a few different types.
Want to know the science behind it?
The Science: Nucleation and Growth of Salt Crystals
Any substance that has a highly ordered structure can be considered as a crystal. Typically, these are solid materials in which the atoms are arranged in a periodic and symmetrical manner.
It’s quite easy to form salt crystals at home from normal table salt dissolved in water… but it’s a bit trickier to form large ones! The salt that we add to our food (in sensible amounts) is made up of tiny little crystals. Rather than turn these small crystals directly into larger ones, we must first dissolve the salt in some water to form a salt solution (boxes 1 and 2), from which we can make larger crystals.
This process is split into two main phases: nucleation and growth. The nucleation stage is the appearance of clusters in which a small number of atoms locally assemble in a manner that resembles the arrangement of atoms found in large crystals (box 3). When additional atoms combine with the small clusters, they eventually grow into larger crystals (box 4).
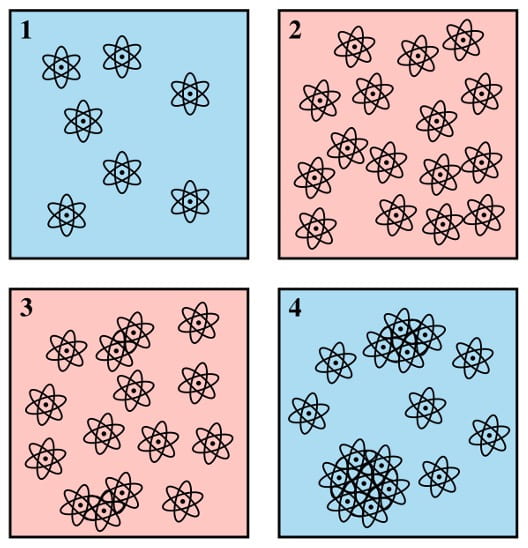
For this process to work, it is important that you have a very high concentration of salt in your solution; the higher the concentration, the more favourable the ordered crystal structure becomes compared to the disordered salt solution. To make your salt solution as concentrated as possible, you must increase the temperature of the water while adding the salt (box 2). This makes what is known as a ‘super-saturated’ solution, which means that the solution contains more salt than it ‘usually’ would (i.e., at equilibrium). It is this supersaturation that is the main driver of the initial nucleation (box 3). If the salt concentration is high enough it becomes more favourable for the salt to form small clusters than to be in solution.
The final stage is the growth of these small clusters into large crystals (box 4). This should be done at room temperature and is a very slow process (it can take days!). Leave your solution in a cool place, away from any disturbances such as washing machines, drum kits, or helicopters.